Your cart is empty
Free shipping on all US orders


Free shipping on all orders
Have you ever wondered why some 70-year-olds complete triathlons while certain 50-year-olds struggle with a single flight of stairs? Why do some faces appear timeless while others seem to age overnight? The answer isn't written in your birth certificate—it's written in your cells.
We've all heard about "biological age" versus "chronological age," but what actually creates this discrepancy? What invisible force determines whether you'll spend your golden years traveling the world or struggling with basic daily tasks? The answer lies in a phenomenon that scientists have only recently begun to fully understand: inflammaging.
Inflammaging—a term combining "inflammation" and "aging"—represents the convergence of two processes that were once thought to be separate. It's the chronic, low-grade inflammation that slowly smolders throughout your body, accelerating every hallmark of aging from wrinkled skin to cognitive decline. Unlike the obvious inflammation you feel when you twist your ankle, inflammaging operates in silence, working behind the scenes to age you from the inside out.
The science of aging has evolved dramatically. We now know that aging isn't just about the passage of time—it's about the accumulation of inflammatory damage. And that damage? It's largely within your control.
To understand inflammaging, we first need to understand inflammation itself—and not all inflammation is your enemy. In fact, the right kind of inflammation is what keeps you alive.
Think of acute inflammation as your body's 911 emergency response system. You slice your finger while cooking—within seconds, a cascade of events begins. Blood vessels at the injury site dilate, increasing blood flow (that's why it turns red). Your capillaries become more permeable, allowing fluid and immune cells to flood the area (that's the swelling). White blood cells arrive like first responders, destroying any bacteria that entered through the wound and clearing away damaged tissue. Meanwhile, other specialized cells begin the repair process, knitting the tissue back together.
This is inflammation at its finest—precise, purposeful, and time-limited. It has a clear beginning (the injury), a middle (the healing process), and a definitive end (complete repair). Within days or weeks, the inflammation resolves completely, and your finger is as good as new. This acute inflammatory response has kept humans alive for millennia, protecting us from infections and enabling tissue repair.
The crucial characteristic of acute inflammation is its self-limiting nature. Your immune system doesn't just know how to start inflammation—it knows exactly when to stop. Special molecules called "pro-resolving mediators" actively shut down the inflammatory process once the threat is neutralized. It strikes a perfect balance of destruction and reconstruction.

Now, imagine that same emergency response system, but the alarm never turns off. The fire trucks keep arriving even though there's no fire. The emergency crew keeps demolishing and rebuilding, but nothing ever fully heals. This is chronic inflammation—specifically, what scientists call "sterile inflammation," where your immune system launches an attack despite the absence of any bacterial or viral invader.
Here's a useful analogy: Acute inflammation is like a controlled campfire. You light it when you need it, it serves a clear purpose (cooking, warmth), and you extinguish it when you're done. Inflammaging, on the other hand, is like faulty electrical wiring smoldering inside your walls. You can't see it, you can't feel it, but it's slowly causing damage 24 hours a day, seven days a week. By the time you notice the symptoms—maybe a scorch mark appears or smoke emerges—significant damage has already occurred.
What makes inflammaging particularly insidious is that it's systemic. Unlike the localized inflammation of a sprained ankle, this low-grade inflammation affects every organ system simultaneously. Your brain, heart, blood vessels, muscles, skin, and joints are all bathed in a constant soup of inflammatory molecules. The levels aren't high enough to cause acute symptoms, but they're more than sufficient to accelerate aging across every system in your body.
The immune cells that should be resting remain in a state of constant, low-level activation. Inflammatory proteins called cytokines circulate through your bloodstream. Your body exists in a perpetual state of "defense mode," expending tremendous energy fighting phantom threats—energy that should be directed toward maintenance, repair, and regeneration.
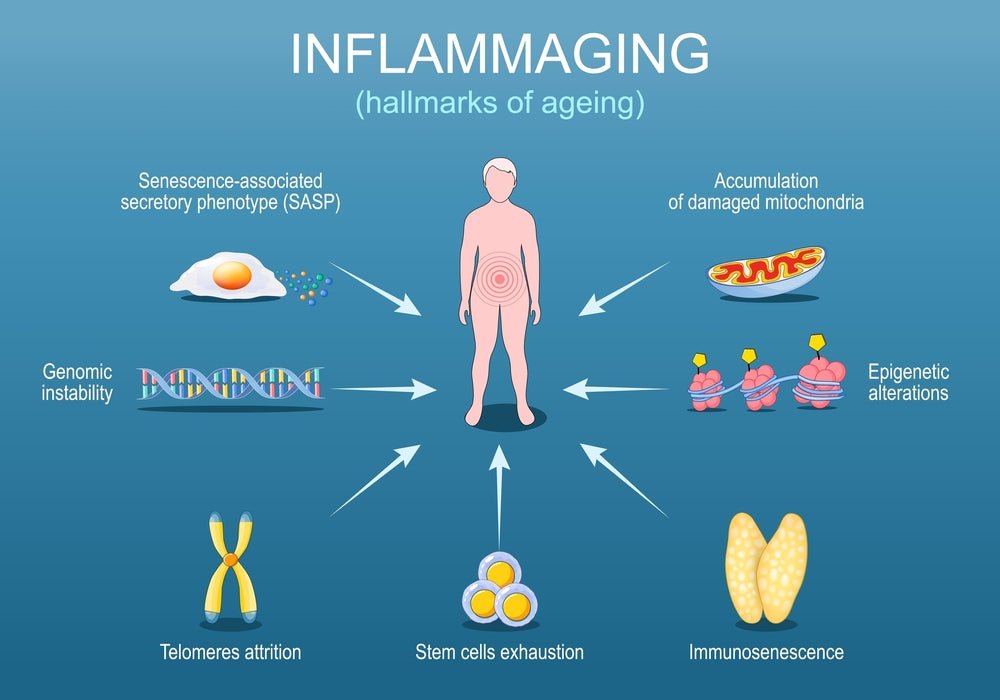
Understanding what drives inflammaging requires us to look under the hood of cellular aging. Four major mechanisms create this self-perpetuating cycle of chronic inflammation, and they all interact and amplify each other in destructive ways.
Your immune system is like a sophisticated military force—but imagine what happens when that army ages. Soldiers retire, training protocols degrade, and the new recruits aren't as well-prepared as the veterans. This is immunosenescence: the gradual deterioration of immune function that occurs with age.
When you're young, your immune system displays remarkable precision. It can distinguish between "self" and "non-self" with incredible accuracy, attacking only legitimate threats while leaving your own healthy tissues alone. But as you age, this precision erodes. The immune system becomes like an exhausted, overworked security guard who, after years on the job, starts getting jumpy and paranoid—seeing threats that aren't there and occasionally attacking innocent bystanders.
The thymus gland, which produces fresh T-cells (crucial immune cells), begins to shrink after puberty and is barely functional by age 60. The T-cells you do have become less diverse, meaning you have fewer varieties of immune cells capable of responding to different threats. Meanwhile, other immune cells called macrophages begin producing more inflammatory cytokines as a default setting, even without provocation.
This loss of precision means your immune system increasingly fails to recognize cellular debris, damaged proteins, and senescent cells (more on these shortly) as "self." Instead, it treats them as foreign invaders, launching inflammatory attacks against your own tissues. The irony is devastating: the very system designed to protect you becomes a source of chronic damage.
One of the most fascinating discoveries in aging research involves what scientists call "zombie cells"—cells that should be dead but refuse to die. The technical term is senescent cells, and understanding them is crucial to understanding inflammaging.

Throughout your life, your cells divide to replace damaged or dead cells. But cells can't divide forever—they have a built-in limit, based on the shortening of protective caps on chromosomes called telomeres.
When cells reach this limit, or when they accumulate sufficient DNA damage, they're supposed to either repair themselves or activate a self-destruct program called apoptosis.
Senescent cells take a third option: they stop dividing but refuse to die. They just sit there, taking up space and consuming resources. While they're no longer performing their original function, they're extremely metabolically active—but not in a good way.
Here's where the zombie analogy becomes particularly apt. These cells secrete a toxic cocktail of inflammatory proteins, growth factors, and enzymes collectively called SASP—the Senescence-Associated Secretory Phenotype. Think of SASP as the zombie bite in a horror movie. These inflammatory factors spread to neighboring healthy cells, inducing inflammation and sometimes even converting those healthy cells into senescent cells. One zombie creates more zombies, which create still more zombies.
The SASP factors include interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α)—all potent inflammatory molecules. They also secrete enzymes called matrix metalloproteinases that literally digest the structural proteins around them, weakening tissues and contributing to visible aging like wrinkles and joint deterioration.
When you're young, your immune system efficiently clears senescent cells almost as quickly as they form. But with immunosenescence, this clearance system fails, and zombie cells accumulate exponentially. By middle age, you might have billions of these cells lurking in your tissues, each one pumping out inflammatory signals 24/7. This is one of the primary drivers of inflammaging.
Your cells are constantly producing waste. Proteins fold incorrectly, cellular components break down, and damaged organelles need disposal. To handle this, cells have an elegant waste management system called autophagy—literally "self-eating."
Damaged proteins and organelles get tagged for disposal, wrapped up in specialized membranes called autophagosomes, and delivered to lysosomes—the cell's waste processing centers—where they're broken down into component parts that can be reused. It's remarkably efficient, allowing cells to maintain a clean, functional interior while recycling valuable molecular building blocks.
But here's the problem: autophagy declines with age. The recycling trucks come less frequently, the processing plants slow down, and garbage begins to pile up. Misfolded proteins aggregate into clumps. Damaged mitochondria (the power plants of cells) accumulate instead of being recycled. Lipofuscin—a yellowish-brown pigment that's essentially cellular garbage—builds up, particularly visible as "age spots" on skin.
This accumulated debris triggers inflammation through multiple pathways. Your immune system, encountering these abnormal protein aggregates and cellular junk, mistakes them for foreign invaders—bacteria or viruses that need to be attacked. Immune cells infiltrate tissues and release inflammatory cytokines in response to what they perceive as a threat. Additionally, some of these accumulated proteins can directly activate inflammatory pathways within cells.
The decline in autophagy is influenced by multiple factors: mTOR (a cellular growth sensor) remains chronically active as we age, suppressing autophagy; cellular energy sensors become less sensitive; and the lysosomes themselves become less efficient at breaking down waste. The result is a vicious cycle where reduced cleaning capacity leads to more garbage, which triggers more inflammation, which further impairs the cleaning systems.
Mitochondria are the tiny power plants inside your cells, converting nutrients into ATP—the energy currency your cells use for everything from muscle contraction to neurotransmitter production. Each cell contains hundreds to thousands of mitochondria, and they're absolutely critical for life.

But mitochondria have a dark side: they're also one of the primary sources of free radicals—unstable molecules that damage cellular components. When mitochondria are young and healthy, they produce ATP cleanly and efficiently, with minimal free radical leakage. But as they age, they become like old engines running roughly, producing more exhaust (free radicals) per unit of useful work (ATP).
These leaked free radicals damage nearby cellular components, including the mitochondria's own DNA. Since mitochondria have their own small genome (inherited only from your mother), they're particularly vulnerable to this damage. Damaged mitochondria become even less efficient, leaking still more free radicals in a self-reinforcing downward spiral.
When cells detect signs of mitochondrial dysfunction—damaged mitochondrial DNA floating in the cytoplasm, excess reactive oxygen species, or depleted ATP levels—they activate this inflammasome. Once activated, NLRP3 triggers the production and release of potent inflammatory molecules, particularly interleukin-1β (IL-1β) and interleukin-18 (IL-18).
All four of these mechanisms—immunosenescence, cellular senescence, autophagy failure, and mitochondrial dysfunction—interconnect and amplify each other. Mitochondrial dysfunction promotes cellular senescence. Senescent cells secrete factors that impair autophagy in neighboring cells. Failed autophagy leads to more damaged mitochondria. And immunosenescence prevents the clearance of senescent cells. It's a perfect storm of aging, with inflammation at the center of the vortex.
The cruelest aspect of inflammaging is that it operates silently for years—even decades—before symptoms appear. You don't feel it happening. There's no pain, no obvious warning signs. The inflammation levels are too low to trigger noticeable symptoms, yet high enough to cause cumulative damage.
During this "silent phase," inflammatory molecules are slowly degrading your tissues, disrupting cellular communication, and accelerating aging across every organ system. By the time symptoms become apparent, significant damage has already occurred. But here's what that damage looks like when it finally surfaces:
Your skin provides the most visible evidence of inflammaging. That youthful, plump appearance comes from an underlying scaffold of collagen and elastin—structural proteins that give skin its firmness and elasticity. When you're young, specialized cells called fibroblasts continuously produce fresh collagen and elastin to maintain this scaffold.

But chronic inflammation releases enzymes called matrix metalloproteinases (MMPs) that digest collagen and elastin faster than fibroblasts can replace them. Imagine a building where the demolition crew works faster than the construction crew—eventually, the structure weakens and collapses. In skin, this manifests as wrinkles, sagging, and loss of that youthful bounce.
Inflammation also impairs the function of fibroblasts themselves, reducing their ability to produce new structural proteins. Additionally, inflammatory molecules break down hyaluronic acid—the molecule responsible for skin hydration and that dewy, plump appearance. The result is thinner, drier, more fragmented skin that shows every line and fold.
The same inflammatory processes also affect wound healing. When you're young, cuts and scrapes heal quickly. With inflammaging, that same injury heals more slowly, leaves more obvious scars, and may even become chronically inflamed. This is why older skin is simultaneously more fragile and slower to repair.
While we can see inflammaging's effects on skin, its impact on the brain may be even more consequential. This is the zone of neuroinflammation—chronic activation of the brain's immune cells, called microglia.
Microglia normally act as the brain's maintenance crew, clearing away debris, pruning unnecessary neural connections, and responding to injuries or infections. But with aging, microglia become chronically activated, existing in a state of constant inflammatory alert. Instead of helpful maintenance, they begin releasing inflammatory cytokines that damage neurons and disrupt the delicate synaptic connections that enable thought, memory, and consciousness.
The early signs of neuroinflammation are subtle: brain fog, difficulty concentrating, slower processing speed, and problems with word recall. These symptoms are often dismissed as "normal aging," but they reflect inflammatory damage to neural networks. As neuroinflammation persists, it contributes to the accumulation of toxic proteins like beta-amyloid and tau—hallmarks of neurodegenerative decline.
Inflammation also disrupts the blood-brain barrier, the selective filter that protects the brain from potentially harmful substances in the bloodstream. A leaky blood-brain barrier allows inflammatory molecules and immune cells to enter brain tissue where they don't belong, further amplifying neuroinflammation. This creates another vicious cycle: inflammation damages the barrier, the damaged barrier permits more inflammation, which causes more barrier damage.
For decades, cardiovascular disease was primarily blamed on cholesterol and lipids. While these factors still matter, we now understand that inflammation is equally—if not more—important in determining cardiovascular risk.

Chronic inflammation doesn't merely contribute to plaque formation; it makes existing plaques unstable and prone to rupture. The inflammatory cells that infiltrate arterial plaques secrete enzymes that digest the fibrous cap covering the plaque. When this cap becomes thin enough, it can rupture, releasing the plaque's contents into the bloodstream. This triggers massive blood clot formation—the immediate cause of most heart attacks and many strokes.
Inflammation also stiffens arterial walls, reducing their ability to dilate and constrict in response to changing blood flow demands. This contributes to hypertension (high blood pressure), which further damages vessel walls in yet another destructive feedback loop. The endothelium—the delicate inner lining of blood vessels—becomes dysfunctional under chronic inflammatory assault, losing its ability to produce nitric oxide, a crucial molecule for vascular health.
Even the heart muscle itself is affected. Chronic low-grade inflammation can lead to subtle fibrosis (scarring) of heart tissue, reducing the heart's pumping efficiency and contributing to heart failure over time. This process is so gradual that many people don't realize their heart function is declining until significant damage has occurred.
Sarcopenia—the progressive loss of muscle mass and strength with aging—was long considered an inevitable consequence of getting older. We now know that inflammaging plays a major causative role.
Inflammatory cytokines, particularly TNF-α and IL-6, directly interfere with muscle protein synthesis. Even when you consume adequate protein and amino acids, these inflammatory signals prevent your muscles from using those building blocks efficiently. It's like having all the materials for construction but someone keeps stealing them before your workers can use them.
Simultaneously, inflammation accelerates muscle protein breakdown. The inflammatory environment activates proteolytic pathways—cellular systems that dismantle proteins. The result is a negative protein balance: you're breaking down muscle faster than you can build it, leading to progressive muscle loss.
This matters profoundly for longevity and quality of life. Muscle isn't just for strength and appearance—it's a metabolic organ that regulates blood sugar, produces anti-inflammatory signaling molecules, and serves as a crucial protein reserve for immune function and healing. Loss of muscle mass is strongly predictive of mortality, hospitalization, and loss of independence in older adults.
The inflammation-muscle connection becomes a vicious cycle: inflammation causes muscle loss, but muscle tissue itself produces anti-inflammatory molecules when it contracts. Less muscle means less anti-inflammatory capacity, allowing inflammation to increase further, causing more muscle loss. Breaking this cycle requires addressing inflammation while simultaneously maintaining muscle through resistance exercise.
Your diet represents one of your most powerful tools for controlling inflammaging—for better or worse. Every meal you eat either adds fuel to the inflammatory fire or helps extinguish it. The modern Western diet, unfortunately, does mostly the former.
Our genes evolved over millions of years in an environment of whole, unprocessed foods: wild game, fish, foraged plants, seasonal fruits, nuts, and seeds. Our immune systems and metabolic machinery are calibrated for this ancestral dietary pattern. But in the last 100 years—a blink of an eye in evolutionary terms—our diet has undergone a radical transformation.
Today, the average person consumes ultra-processed foods that didn't exist until recently: refined sugars, inflammatory seed oils, artificial additives, and "food-like substances" engineered for profit rather than nourishment. Our ancient genes encounter these modern inputs and respond with confusion and alarm—triggering inflammatory cascades because these substances resemble, at a molecular level, signals of cellular damage or invasion.
This evolutionary mismatch is a primary driver of the inflammaging epidemic. We're essentially asking our Stone Age biology to process Space Age chemistry, and the result is chronic inflammation.
Refined Sugars and Processed Carbohydrates: When you consume refined sugar or processed carbs, your blood glucose spikes rapidly. Your pancreas responds by flooding your bloodstream with insulin to shuttle that glucose into cells. But chronically elevated insulin doesn't just regulate blood sugar—it also activates inflammatory pathways, particularly through a protein called NF-κB, the master regulator of inflammatory gene expression.

Additionally, high blood sugar leads to a process called glycation, where glucose molecules stick to proteins and fats, creating harmful compounds called advanced glycation end products (AGEs). These AGEs are recognized by immune cells as abnormal and trigger inflammatory responses. They also directly damage tissues—contributing to arterial stiffening, kidney damage, and skin aging.
The worst offenders are sugary beverages, candy, baked goods, and foods made with refined white flour. These deliver massive glucose loads with minimal nutritional benefit, creating inflammatory spikes throughout the day.
Seed Oils (High Omega-6 Content): Not all fats are created equal when it comes to inflammation. Omega-6 fatty acids, particularly linoleic acid, are precursors to inflammatory molecules called prostaglandins and leukotrienes. While we need some omega-6 fats (they're technically essential), the modern diet provides them in extreme excess—primarily through industrial seed oils like soybean, corn, canola, and sunflower oil.
Our ancestors consumed omega-6 and omega-3 fatty acids in roughly a 1:1 to 4:1 ratio. Modern Americans often consume them in a 20:1 ratio, or even higher. This massive imbalance skews our inflammatory pathways toward inflammation, as the enzyme systems that process these fats get overwhelmed with omega-6 precursors, churning out inflammatory molecules while producing insufficient anti-inflammatory compounds.
These oils are ubiquitous in the modern food supply—in salad dressings, mayonnaise, packaged snacks, restaurant foods, and baked goods. Minimizing their intake is one of the highest-impact dietary interventions for reducing inflammaging.
Ultra-Processed Foods and Additives: Foods that come in boxes, bags, and wrappers often contain a cocktail of additives—emulsifiers, preservatives, artificial colors, and flavorings—that can trigger inflammatory responses. Many of these compounds disrupt the gut microbiome, damage the intestinal barrier, or directly activate immune cells.
Emulsifiers like carboxymethylcellulose and polysorbate-80, commonly used to improve texture in processed foods, have been shown to degrade the mucus layer protecting the intestinal lining, allowing bacteria and food particles to contact immune cells in the gut wall directly—triggering inflammation. The more processed the food, generally the more inflammatory potential it carries.
The Omega-3 Ratio: While omega-6 fats drive inflammation, omega-3 fatty acids—particularly EPA and DHA found in fatty fish—are potently anti-inflammatory. These fats are incorporated into cell membranes throughout your body, where they serve as precursors to specialized pro-resolving mediators—molecules that actively shut down inflammatory responses and promote healing.
EPA and DHA also compete with omega-6 fats for the same enzyme systems, meaning that when you increase omega-3 intake, you automatically reduce inflammatory molecule production. The best sources are cold-water fatty fish: salmon, mackerel, sardines, herring, and anchovies. For plant-based sources, flaxseeds, chia seeds, and walnuts provide ALA, an omega-3 precursor, though the conversion to EPA and DHA is inefficient—typically only 5-10%.
To optimize your inflammatory balance, aim for omega-3-rich foods at least 3-4 times per week, while simultaneously reducing omega-6 seed oils. This two-pronged approach rebalances your inflammatory pathways from the ground up.
Polyphenols: Have you ever noticed that the most nutrient-dense foods are also the most colorful? Those vibrant reds, purples, blues, and greens come from polyphenols—plant compounds that protect against inflammaging through multiple mechanisms.
Polyphenols act as antioxidants, neutralizing free radicals before they can damage cellular components and trigger inflammatory pathways. But they also work at a deeper level, influencing gene expression to reduce inflammatory cytokine production while enhancing antioxidant enzyme systems. They inhibit NF-κB activation, modulate NLRP3 inflammasome activity, and even improve mitochondrial function.

Different polyphenols are found in different foods: resveratrol in grapes and berries, curcumin in turmeric, EGCG in green tea, quercetin in onions and apples, and anthocyanins in blue and purple foods. The key is diversity—consuming a wide variety of colorful plant foods ensures you're getting the full spectrum of these protective compounds.
The Gut Barrier and Short-Chain Fatty Acids: One of the most important anti-inflammatory strategies involves feeding your gut microbiome properly. The bacteria residing in your intestines aren't passive passengers—they're active participants in your immune function and inflammatory status.
When you consume fiber—particularly fermentable fiber from vegetables, fruits, legumes, and whole grains—your gut bacteria ferment it, producing short-chain fatty acids (SCFAs) like butyrate, propionate, and acetate. These SCFAs are remarkably powerful anti-inflammatory molecules.
Butyrate, in particular, serves as the primary fuel source for the cells lining your colon, keeping them healthy and maintaining the integrity of your intestinal barrier. A healthy gut barrier is crucial because when it becomes "leaky," bacterial components called lipopolysaccharides (LPS) can enter your bloodstream, triggering systemic inflammation. Butyrate prevents this leakage while also directly influencing immune cell function to reduce inflammatory responses.
Additionally, SCFAs can travel through your bloodstream to influence inflammation in distant organs, including the brain. They help regulate immune cell development and function, promoting anti-inflammatory regulatory T-cells while reducing pro-inflammatory immune cell populations.
To maximize SCFA production, consume plenty of fiber-rich whole foods: leafy greens, cruciferous vegetables, legumes, berries, and whole grains. Fermented foods like sauerkraut, kimchi, and kefir provide both beneficial bacteria and their anti-inflammatory products.
Let's address the elephant in the room: we all know we should eat more vegetables. We've heard it since childhood. But here's the uncomfortable truth—the overwhelming majority of people don't consume nearly enough plant diversity to meaningfully impact inflammaging.
The USDA recommends five to nine servings of fruits and vegetables daily, with an emphasis on variety to capture different phytonutrients. Yet data consistently shows that less than 10% of Americans meet even the minimum recommendations. The average American consumes perhaps two to three servings per day, often with minimal variety—maybe some iceberg lettuce on a sandwich, a banana, and if we're lucky, a side of broccoli at dinner.
But it's not just about quantity of what you consume—it's about the diversity of phytonutrients, too. Different colored vegetables provide different protective compounds: carotenoids in orange and red vegetables, glucosinolates in cruciferous vegetables, polyphenols in berries, allicin in garlic. To truly combat inflammaging, you need exposure to this wide spectrum of protective compounds regularly.
The reality is that consistently shopping for, storing, preparing, and consuming this variety is incredibly challenging for most people juggling work, family, and life's demands. Fresh vegetables spoil quickly, require preparation time, and can be expensive. This is the phytonutrient gap—the difference between what we need and what we realistically consume.
This is where Field of Greens enters as a practical solution—not as a substitute for vegetables, but as an insurance policy that guarantees you're getting a research-backed serving of diverse, whole-food nutrition every single day.
Field of Greens isn't your typical greens powder filled with cheap fillers and grass clippings. It's a carefully formulated blend of organic fruits and vegetables that's been dehydrated and ground into a powder, preserving the full spectrum of phytonutrients, fiber, and bioactive compounds. Each serving delivers the equivalent of multiple servings of diverse vegetables and fruit in a form that's actually convenient and tasty enough to use consistently.
But here's what separates Field of Greens from the hundreds of other supplements making promises: it's backed by real clinical research.
Field of Greens underwent validation at Auburn University in a proper clinical study—not a paid testimonial or marketing fluff, but actual scientific methodology with measurable outcomes. This distinction matters enormously in the supplement industry, where most products make bold claims without any clinical backing whatsoever.
The Methodology: Researchers at Auburn University conducted a controlled study examining the metabolic effects of daily Field of Greens consumption. Participants were provided with the supplement and monitored over a specific period, with various health markers measured at baseline and at study conclusion. The study focused on markers directly relevant to inflammaging and age-related metabolic decline.
Key Findings:
Metabolic Health Improvements: One of the most significant findings involved improvements in metabolic health markers. Participants showed improvements in key indicators of metabolic function—the efficiency with which your cells process nutrients and generate energy. This matters for inflammaging because metabolic dysfunction and inflammation are intimately linked. Poor metabolic health drives inflammation, while inflammation impairs metabolism. Breaking this cycle is crucial for healthy aging.
Oxidative Stress Reduction: Perhaps most relevant to inflammaging, the study demonstrated measurable reductions in oxidative stress markers. Oxidative stress—the imbalance between free radical production and antioxidant defenses—is one of the primary triggers of chronic inflammation. The accumulated free radicals from mitochondrial dysfunction, discussed earlier, damage cellular components and activate inflammatory pathways.
By providing a concentrated source of diverse phytonutrients with antioxidant properties, Field of Greens demonstrably reduced this oxidative burden. Participants showed improvements in markers indicating their bodies had more robust antioxidant defenses—they were better equipped to neutralize free radicals before they could trigger inflammatory cascades. This is exactly the kind of intervention needed to address the root causes of inflammaging discussed previously.
Nitric Oxide and Vascular Health: The study also revealed improvements in nitric oxide production and vascular function. Nitric oxide is a crucial signaling molecule produced by the endothelium (the inner lining of blood vessels). It causes blood vessels to dilate, improving blood flow and reducing blood pressure. It also has anti-inflammatory properties and helps prevent the arterial damage that leads to cardiovascular disease.
Many vegetables in Field of Greens, particularly beets and leafy greens, are rich in nitrates that your body converts to nitric oxide. The Auburn study confirmed that Field of Greens consumption led to improvements in markers of vascular health—suggesting better blood flow, enhanced delivery of nutrients and oxygen to tissues, and reduced cardiovascular inflammation.
Let's connect these findings back to the mechanisms of inflammaging we discussed earlier. Remember the four horsemen: immunosenescence, cellular senescence, autophagy failure, and mitochondrial dysfunction. Field of Greens addresses multiple aspects of this destructive quartet.
The reduction in oxidative stress directly impacts mitochondrial health. By neutralizing free radicals, the diverse phytonutrients in Field of Greens reduce the oxidative damage that impairs mitochondrial function and triggers NLRP3 inflammasome activation. Healthier mitochondria produce energy more efficiently with less inflammatory byproduct.
The polyphenols and other bioactive compounds help modulate inflammatory signaling pathways, reducing the chronic activation of NF-κB and other pro-inflammatory transcription factors. This means fewer inflammatory cytokines circulating in your system, less inflammatory pressure on tissues, and reduced stimulus for cellular senescence.
The improved vascular function has widespread anti-inflammatory benefits. Better blood flow means improved delivery of nutrients and oxygen to tissues and more efficient removal of inflammatory waste products. The anti-inflammatory properties of nitric oxide itself help protect the endothelium from inflammatory damage.
Additionally, many of the compounds in Field of Greens support autophagy and cellular cleaning processes. Certain polyphenols have been shown to activate autophagy pathways, essentially helping your cells maintain better housekeeping—reducing the accumulation of cellular debris that triggers inflammatory responses.
Field of Greens essentially provides a daily infusion of the exact compounds your body needs to fight inflammaging—the polyphenols that neutralize free radicals, the nitrates that support vascular health, the fiber that feeds beneficial gut bacteria, and the diverse phytonutrients that modulate inflammatory gene expression. And unlike most supplements, it has clinical data proving it actually works as intended.
While nutrition forms the foundation of an anti-inflammaging strategy, several lifestyle factors can either amplify or undermine your dietary efforts. These factors influence inflammation through distinct mechanisms, each backed by robust scientific evidence.
We've all heard that exercise is good for us, but the mechanism by which it combats inflammaging is fascinating and only recently understood. When you exercise—particularly when your skeletal muscles contract—those muscles don't just generate movement; they function as endocrine organs, secreting signaling proteins called myokines into your bloodstream.
Myokines are anti-inflammatory messenger molecules that travel throughout your body, influencing immune function, metabolic health, and even brain function. Key myokines like IL-6 (yes, the same molecule that can be pro-inflammatory in other contexts acts as anti-inflammatory when released during exercise), IL-10, and irisin actively reduce systemic inflammation, improve insulin sensitivity, and promote the clearance of senescent cells.
This is why exercise is often called "polypharmacy"—it simultaneously addresses multiple aspects of inflammaging through a single intervention. But not all exercise affects inflammation equally.
The Sweet Spot: Zone 2 Cardio: Zone 2 cardio refers to moderate-intensity exercise where you're working just hard enough to elevate your heart rate but can still maintain a conversation. This typically corresponds to about 60-70% of your maximum heart rate. What makes Zone 2 special for inflammaging is its effect on mitochondria.
Extended Zone 2 exercise stimulates mitochondrial biogenesis—the production of new, healthy mitochondria. Remember that mitochondrial dysfunction is one of the core drivers of inflammaging, with damaged mitochondria leaking free radicals and activating the NLRP3 inflammasome. By promoting the production of new, efficient mitochondria while improving the quality control that removes damaged ones, Zone 2 training directly addresses this inflammatory driver.
Aim for 150-180 minutes of Zone 2 work per week—this could be brisk walking, cycling, swimming, or any sustained moderate activity.
Resistance Training: While cardio builds mitochondrial health, resistance training is crucial for combating the inflammatory muscle loss of sarcopenia. When you lift weights or perform bodyweight exercises, you create micro-damage in muscle fibers. In response, your body does more than just repair the damage—it overcompensates, building more and stronger muscle tissue.
More muscle mass means more myokine-producing tissue, giving you a larger anti-inflammatory capacity. Studies show that individuals with higher muscle mass have lower levels of inflammatory markers and better metabolic health, even independent of overall body weight.
Resistance training also improves insulin sensitivity, reducing the inflammatory cascade triggered by elevated insulin and blood glucose. Aim for at least two to three resistance training sessions per week, targeting all major muscle groups.
Of course, you also need to ensure you’re meeting your protein needs, which can be supported via use of The Brickhouse Whey; highly bioavailable and absorbable form of fermented whey to ensure you get the most out of your diet.
Stress and inflammation are intimately connected through the HPA axis—the hypothalamic-pituitary-adrenal axis—your body's central stress response system. When you experience stress, your hypothalamus signals your pituitary gland, which signals your adrenal glands to release cortisol and other stress hormones.
In the short term, cortisol is actually anti-inflammatory—it's the body's way of preventing an excessive immune response. But here's where things go wrong: chronic stress leads to chronically elevated cortisol, and over time, your immune cells develop glucocorticoid resistance. They stop responding to cortisol's "stop" signals.
Imagine a car alarm that goes off so often that everyone in the neighborhood learns to ignore it. That's what happens with chronic cortisol exposure. Your immune cells become desensitized to cortisol's anti-inflammatory effects, but the cortisol itself continues having other problematic effects—disrupting sleep, increasing blood sugar, breaking down muscle tissue, and impairing healing.
The result is that chronic stress creates a situation where inflammation can run unchecked because the very hormone that should be controlling it has lost its effectiveness. Studies show that individuals under chronic stress have higher levels of inflammatory markers like CRP and IL-6, even when controlling for other factors.
Actionable Stress Reduction Strategies
Box Breathing: This simple technique—inhaling for four counts, holding for four counts, exhaling for four counts, holding for four counts—activates the parasympathetic nervous system, the "rest and digest" counterbalance to the stress response. Just five minutes of box breathing can measurably reduce cortisol levels and shift your nervous system out of fight-or-flight mode.

Nature Exposure: Research shows that spending time in natural environments—forests, parks, beaches—reduces cortisol levels and inflammatory markers. Even 20 minutes of forest bathing (simply being present in nature) has demonstrable anti-inflammatory effects. Nature exposure seems to recalibrate the stress response system, providing a buffer against daily stressors.
Digital Detox: The constant stream of notifications, news, and social media comparison triggers repeated stress responses throughout the day. Each notification spike activates your stress system briefly—not enough to notice consciously, but enough to keep cortisol elevated. Implementing regular digital detox periods—even just an hour before bed or a few hours on weekends—allows your stress system to fully reset.
If you could only choose one lifestyle intervention for inflammaging, sleep might be the most impactful. The connection between sleep and inflammation is profound and bidirectional: inflammation disrupts sleep, and poor sleep drives inflammation.
During deep sleep, something remarkable happens in your brain. The glymphatic system—discovered only in the last decade—activates like a nighttime cleaning crew. Your brain cells literally shrink by about 60%, increasing the space between them. Cerebrospinal fluid then flows through these expanded spaces, washing away accumulated metabolic waste products, including beta-amyloid (the protein that aggregates in Alzheimer's disease) and inflammatory molecules.
This cleaning process happens almost exclusively during the deep, slow-wave stages of sleep. If you don't get sufficient deep sleep, these inflammatory toxins accumulate. Studies using brain imaging show that even a single night of poor sleep leads to increased beta-amyloid accumulation and elevated brain inflammation.
The inflammatory consequences of sleep deprivation extend beyond the brain. A single night of inadequate sleep increases inflammatory markers throughout the body. Chronic sleep restriction leads to persistent elevation of IL-6, TNF-α, and CRP. It impairs insulin sensitivity, disrupts hunger hormones leading to overeating, and accelerates cellular senescence.
Sleep Hygiene for Anti-Inflammatory Sleep
Aim for 7-9 hours of sleep per night, with consistent sleep and wake times—even on weekends. This consistency helps regulate your circadian rhythm, which directly influences inflammatory molecule production throughout the day.
Keep your bedroom cool (65-68°F), dark, and quiet. Temperature drop is a crucial signal for deep sleep initiation, and light exposure at night suppresses melatonin—which has direct anti-inflammatory properties beyond its sleep-promoting effects.
Limit blue light exposure from screens at least an hour before bed. Blue wavelengths suppress melatonin more powerfully than other wavelengths, disrupting your sleep architecture and reducing deep sleep stages—exactly when glymphatic cleaning occurs.
"Is it too late to start if I'm already over 60?"
This is perhaps the most common question, and the answer is unequivocally: it's absolutely not too late. While it's true that the earlier you start addressing inflammaging, the better your outcomes, the human body retains remarkable plasticity even in later decades of life.
Multiple studies demonstrate that anti-inflammatory lifestyle interventions produce measurable benefits even when started in your 60s, 70s, or beyond. One landmark study showed that previously sedentary adults starting resistance training in their 70s gained significant muscle mass and strength, with corresponding reductions in inflammatory markers—within just 12 weeks. Another study found that dietary changes reduced CRP levels and improved metabolic markers in people over 65 to a similar degree as younger participants.
The benefits aren't just biochemical—they translate to real-world outcomes. Studies show that lifestyle interventions started in later life still reduce the risk of cardiovascular events, improve cognitive function, and enhance independence and quality of life. Your cells are constantly turning over, and every healthy choice you make influences those new cells.
Think of it this way: while you can't undo decades of accumulated damage overnight, you can immediately stop adding more damage and start providing your body with the resources it needs to repair and maintain itself. The inflammatory burden begins decreasing within days to weeks of implementing anti-inflammatory strategies. Starting today is infinitely better than waiting another year.
"How do I know if I have inflammaging? Are there tests?"
Inflammaging operates silently for years, but there are measurable biomarkers if you want objective data. The most accessible and clinically relevant test is high-sensitivity C-Reactive Protein (hs-CRP), a blood test that measures a protein produced by your liver in response to inflammation.
CRP levels below 1 mg/L are considered low risk, 1-3 mg/L indicate moderate risk, and above 3 mg/L suggest high inflammation. This test is inexpensive, widely available, and validated as a predictor of cardiovascular risk and overall inflammatory burden. You can request it from your doctor as part of routine bloodwork, and many direct-to-consumer lab services offer it.
Other useful markers include IL-6 (directly measures a key inflammatory cytokine), TNF-α, and homocysteine (an amino acid that increases with inflammation). A complete metabolic panel showing elevated glucose, insulin resistance markers, or liver enzymes can also indicate inflammatory stress.
However, you don't necessarily need testing to benefit from anti-inflammatory strategies. Since these interventions (whole foods diet, exercise, stress management, adequate sleep) are beneficial regardless of your current inflammatory status and carry virtually no downside risk, you can implement them based on the universal understanding that inflammaging affects everyone with age.
"Is coffee inflammatory?"
This question reveals important nuance: coffee itself, consumed appropriately, is actually anti-inflammatory. High-quality coffee is rich in polyphenols—particularly chlorogenic acid—that have demonstrated anti-inflammatory properties. Studies consistently show that moderate coffee consumption (3-4 cups daily) is associated with lower inflammatory markers and reduced risk of various age-related diseases.
The problems arise with what people add to coffee and how they consume it. A large dessert-like coffee beverage loaded with sugar, artificial sweeteners, and inflammatory seed oils (in most coffee creamers) becomes pro-inflammatory despite the coffee itself. Similarly, drinking coffee late in the day can disrupt sleep quality—and as we've discussed, poor sleep is strongly pro-inflammatory.
The sweet spot: Consume high-quality organic coffee (to minimize pesticide exposure) in the morning and early afternoon, with minimal additives. Black coffee, coffee with a small amount of whole milk or cream, or coffee with unsweetened alternatives are all fine. Avoid the sweetened, flavored coffee beverages that are essentially desserts in disguise.
"Does fasting help with inflammaging?"
Fasting—particularly intermittent fasting or periodic extended fasts—can be a powerful tool for combating inflammaging, primarily through its effects on autophagy. Remember that autophagy is your cellular recycling system that declines with age, allowing damaged components and cellular debris to accumulate and trigger inflammation.
When you fast, particularly for periods of 16 hours or longer, you shift your cellular metabolism away from growth and toward maintenance. The absence of incoming nutrients sends signals that activate autophagy pathways, essentially telling cells: "There's no new material coming in, so let's clean house and recycle what we have."
This activation of autophagy helps clear senescent cells, damaged mitochondria, misfolded proteins, and other cellular debris that drive inflammaging. Studies show that fasting reduces inflammatory markers, improves mitochondrial health, and may even reduce the burden of senescent "zombie cells."
The most accessible approach is time-restricted eating, typically a 16:8 pattern (fasting for 16 hours, eating within an 8-hour window). This could mean eating your first meal at noon and your last meal by 8 PM. Longer fasts (24-72 hours) may provide additional benefits but should be approached carefully and ideally under medical supervision, especially if you have existing health conditions or take medications.
One important caveat: fasting should support, not replace, adequate nutrition. The goal is to compress your eating window, not to chronically under-eat or become nutrient deficient. During your eating window, focus on nutrient-dense, anti-inflammatory foods.
"Can I just take Ibuprofen every day?"
This is a critical question because it reveals a fundamental misunderstanding of how to address inflammaging. NSAIDs (non-steroidal anti-inflammatory drugs) like ibuprofen work by blocking the production of inflammatory molecules called prostaglandins. While this provides relief from acute inflammatory symptoms like pain and swelling, it doesn't address the root causes of inflammaging—and chronic NSAID use carries significant risks.
Long-term NSAID use increases risk of gastrointestinal bleeding, ulcers, kidney damage, and cardiovascular problems. These drugs interfere with the protective prostaglandins in your stomach lining and kidneys, and some evidence suggests they may actually impair the body's natural inflammation resolution processes—preventing inflammation from completing its cycle and properly shutting down.
Compare this to the natural approach: addressing inflammaging through diet, exercise, sleep, and targeted supplementation works by supporting your body's own resolution of inflammation. Rather than blocking inflammatory molecules, you're reducing the triggers that produce them in the first place while enhancing your body's ability to resolve inflammation naturally through specialized pro-resolving mediators.
The drug approach treats symptoms while potentially creating new problems. The lifestyle approach addresses root causes while producing positive side effects: better energy, improved body composition, enhanced cognitive function, and increased resilience. NSAIDs have a place for acute injuries and short-term use, but they're not a solution for chronic inflammaging.
We've journeyed deep into the machinery of aging, from the cellular "zombie" phenomenon to the failing mitochondria that leak inflammatory free radicals. You now understand that aging isn't just about the passage of time—it's about the accumulation of inflammatory damage. And that damage, while insidious, is largely within your control.
Think of inflammaging as a fire smoldering in the walls of your body. For years it burns silently, causing damage you can't see or feel. But eventually, it manifests as wrinkles, brain fog, muscle loss, arterial plaques, and all the visible markers we associate with "getting old." The good news? You now have a comprehensive fire extinguisher.
The hierarchy of intervention is straightforward.
First: Stop throwing fuel on the fire. Every meal of processed foods, every night of poor sleep, every day of chronic stress adds accelerant to the inflammatory fire. Eliminating or minimizing these triggers—refined sugars, seed oils, ultra-processed foods, sleep deprivation, and unmanaged stress—immediately begins reducing your inflammatory burden. You can't out-supplement a terrible lifestyle.
Second: Active cooling. Exercise releases anti-inflammatory myokines, builds mitochondrial health, and combats sarcopenia. Quality sleep activates your glymphatic cleaning system and allows cellular repair processes to complete. Stress management prevents glucocorticoid resistance and keeps your immune system properly calibrated. These active interventions don't just remove fuel—they actively extinguish existing flames.
Third: Reinforcement. This is where strategic nutrition and supplementation like Field of Greens come into play. By providing concentrated, diverse phytonutrients that neutralize free radicals, modulate inflammatory gene expression, and support cellular cleaning processes, you're giving your body additional tools to combat inflammaging. The Auburn University research confirms these aren't just theoretical benefits—they're measurable improvements in the exact markers that determine your inflammatory burden and your biological age.
The science of inflammaging represents one of the most important breakthroughs in our understanding of aging. We're no longer passive victims watching our bodies deteriorate with the passage of time. We now understand the molecular mechanisms driving aging, and we have evidence-based tools to intervene.
Start today. Start small if needed—maybe it's adding Field of Greens to your morning routine, or committing to seven hours of sleep, or taking a 20-minute walk. What matters isn't perfection; it's consistency. The inflammatory fire of aging has been burning for years. It's time to start extinguishing it, one choice at a time, for a longer, healthier, more vibrant life.
References
Cellular Senescence: A Translational Perspective - Kirkland JL, Tchkonia T. (2017). EBioMedicine. https://pubmed.ncbi.nlm.nih.gov/28416161/
Exercise is the Real Polypill - Fiuza-Luces C, et al. (2013). Physiology. https://pubmed.ncbi.nlm.nih.gov/23997192/
The Glymphatic System: A Beginner's Guide - Jessen NA, et al. (2015). Neurochemical Research. https://pubmed.ncbi.nlm.nih.gov/25947369/
Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man - Calder PC. (2017). Biochemical Society Transactions. https://pubmed.ncbi.nlm.nih.gov/28900017/
Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects - Zhang H, Tsao R. (2016). Current Opinion in Food Science. https://doi.org/10.1016/j.cofs.2016.02.002
Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? - Hussain T, et al. (2016). Oxidative Medicine and Cellular Longevity. https://pubmed.ncbi.nlm.nih.gov/27738491/
The Sleep-Immune Crosstalk in Health and Disease - Besedovsky L, et al. (2019). Physiological Reviews. https://pubmed.ncbi.nlm.nih.gov/30920354/